By Kirstin R.W. Matthews, Ph.D.
Fellow in Science and Technology Policy
On July 27, 2020, Moderna Therapeutics started its Phase 3 clinical trial for its vaccine against Covid-19, one of several vaccine candidates
currently being developed. The trial will enroll more than 30,000 participants to determine if there are any side effects to the vaccine and how effective it is against the virus. Effectiveness will be compared to the population as well as trial participants who receive a placebo. Data and results for this large trial are anticipated in early 2021.
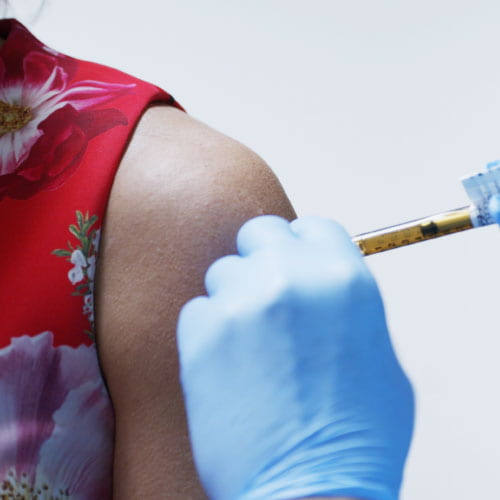
Christene Kimmel, Baker Institute director of development for energy studies and special projects, was the first Houstonian to receive a vaccine in the Phase 3 Moderna trial. In a Q&A with Baker Institute fellow Kirstin Matthews, Kimmel describes her experiences with the trial and why she decided to participate.
How did you learn about the vaccine trial?
On July 5, my family and I had returned home from contactless camping at YMCA Camp Cullen for a week. While we were there, I was scrolling on social media and saw that Moderna’s Phase 2 results were released and Moderna was opening Phase 3 nationwide later in July. I was hopeful that Houston would host a trial location because of all the research that is conducted in the Texas Medical Center (TMC) and that Houston was in the midst of Covid-19 outbreak. That evening, there was a story on the news about trials and I decided to start to gather information to see if this was something I could be interested in undertaking.
Why did you decide to volunteer?
The inspiration for my participation in the trial comes from needing to help and wanting “to make a difference” — as the mosaic reads on Rice University’s Baker Institute for Public Policy building. I, like other Houstonians, have wanted to help from the start of the pandemic. Houstonians pride ourselves on our crisis response, we are a culture of helpers. I was struggling with where and how I could help. Putting my arm out in order to receive the vaccine helps Houston and the world get one step closer to a day where we can all safely hug each other again.
I am an extrovert. I love my job, since I meet people all of the time, shake hands, hug, have conversations over meals or coffees, attend crowded events at the institute and off-campus, etc. My interactions with others as a development officer now carries serious and potentially life-threatening implications. I miss seeing people in person. I miss the energy from conversations. I miss feeling excited about participating in an event or meeting.
It has been hard for me to grapple with how severely my work and personal life have been impacted. The weight of the consequences of our daily actions are disproportionate to the decisions we are used to making. There has never been a time prior to the Covid-19 pandemic that I have had to consider that a simple interaction at the grocery store, a touch to my eyes, nose, or face could affect my health or the health of others. I was pregnant with my daughter during the 2009 swine flu pandemic and I never felt that my life was at risk, as I do now.
I was asked what I wanted for my birthday by my parents back in April (my birthday is in July). At that point, I said a hug. As the days became months, it became apparent that my birthday hug was not going to happen. I needed to figure out how I could help all of us get back to a place where seeing each other in person would not result in illness.
What was the application form/process like?
The initial process was easy. I went to https://houstonfightscovid.com/ and followed the prompts under the “join the fight.” The form was straightforward and accessible. The website was bilingual, which gave me hope that Moderna was actively recruiting a diverse population, especially minority communities disproportionately affected by Covid-19. The application inquired about my basic demographic information and zip codes for where I work and I live. In addition, there was a series of statements to determine my risk status, including if I was a first responder, front-line worker, or worked in place that had a large number of employees; if I had attended religious gatherings, protests, or go shopping more than once a week; if I had children in daycare or guests over to my home; and if I wear a mask and practice social distancing.
I applied on July 12 and had a phone interview on July 16 with a trial recruiter, which took only 15 minutes to determine if I was a viable candidate. After the call, I receive an email with a link to a set of documents including another questionnaire and informed consent forms that needed to be filled out and signed. After those documents were submitted,– I was called back with the news that I had an appointment on July 29th at 8am!,
Through the entire process, a consistent mantra that all involved in the trial have repeated to me is that I can back out at any time, no questions asked. The language is woven into all of the materials that I signed. The speed of these vaccine trials is unprecedented and historic. I know that they are following FDA guidelines and that everyone is under intense scrutiny to quickly deliver a vaccine that can help to protect us. But there is not a chance that I will back out of the trial; I have determined that participation in this trial is better than any birthday gift and as close to a hug as I am going to get in 2020.
Do you have any fears or concerns about participating?
My biggest concern was whether I could find data on the severity of symptoms experienced by Phase 1/2 trial participants. I wanted to figure out if I could physically handle the most extreme set of symptoms, and the impact on my family watching me go through them. I found an article about Ian Haydon in Seattle, Wash., who participated in the Phase 1 trial and received the highest dosage of the trial vaccine, 250 ug. He was very transparent about his experience. He easily tolerated the first dose. However, within 12 hours of the second dose he had a high fever, chills, and was advised to go to the emergency room by the doctors overseeing the trial. Even after reading about his experience, I was still resolute that I would participate in the trial.
The final two references for my decision to enroll were the Baker Institute’s Center for Health and Bioscience’s Blog Series, “Covid-19 by the Numbers,” and the July 15 Phase 1 peer reviewed results that were published in the New England Journal of Medicine. I knew that the Phase 3 trial vaccine dosage was 100 ug. I reviewed the symptom charts and again determined 100% that I wanted to participate in this trial. The only way that I would not participate is if my medical history excluded me from being a viable candidate.
Can you describe what happened on the day of your appointment.
My first appointment was on July 29. However, it was delayed due to issues with access to the necessary medical materials for the trial (not an issue with the quality of the vaccine). I was finally scheduled to receive the vaccine on August 14 at 8:00 a.m.
I was checked in by a nurse who took my temperature. A second nurse took me through my next steps. I filled out additional consent forms (which was long, maybe four pages), provided a list of my medications, and signed a document that explained the blinding of the trial. I had a 50/50 chance of receiving a placebo; I still don’t know if I received the vaccine or the placebo, nor does the clinic or physicians. They also took about eight vials of blood to test for existing antibodies, T-cells and other factors to provide a baseline to determine if the vaccine is effective. Finally, they administered the deep in the sinus Covid-19 test.
After receiving the shot, they asked me to journal via an app on my phone. It prompts me daily to enter data about general wellness, body temperature, medications taken for any reason and to document any pain or symptoms that I relate to the injection.
Do you anticipate changing any of your behavior to test the vaccine?
I have been practicing social distancing with a limited number of people. I would like to socialize with more people, but I am not willing to risk anyone’s health. I miss interacting with my friends and I know that my children miss hanging out with their friends, too. However, I truly only feel safe doing that outside, where I have control of the surfaces that I touch. The Houston heat makes that hard, so we recently installed a misting system in our backyard to make it more comfortable for people to visit outside.
I don’t plan to go anywhere more risky, though. I do not trust the sanitation practices outside of my purview. At this point, I need to be in control of where I sit, eat, all cutlery, plates and glasses. Plenty of my favorite restaurants have meals and cocktail kits to go, so I can support my favorite restaurants by enjoying their meals at my home.
Did you experience any initial side effects in the first 24 hours?
There was soreness at the site, as expected with any vaccine. However, I didn’t get any other symptoms like fever, aches or swelling.
I have been asked if I know, or my guess on, whether I received the vaccine or placebo. There is a part of me that is hoping for symptoms after my second injection, because I feel that would make my contribution to the study complete. I would be kidding myself if I did not admit that if I do not exhibit symptoms after the second shot, I will be disappointed. I have spent the past week reminding myself that my participation in the study, despite what is in the needle, means that I helped to make a difference.
Photo of Kimmel receiving the Covid-19 vaccine courtesy of Texas Center for Drug Development.